

We need to develop alternative potency assays to quantify parameters such as transgene expression, proliferative capacities, phenotype (memory vs. The 51Cr-release assay remains the gold standard to assay cytotoxicity however, it is hazardous and almost untransferable due to 51Cr radioactivity. To date, measuring cytotoxicity is the preferred assay to assess CAR T-cell potency, although standardized assays are currently unavailable.
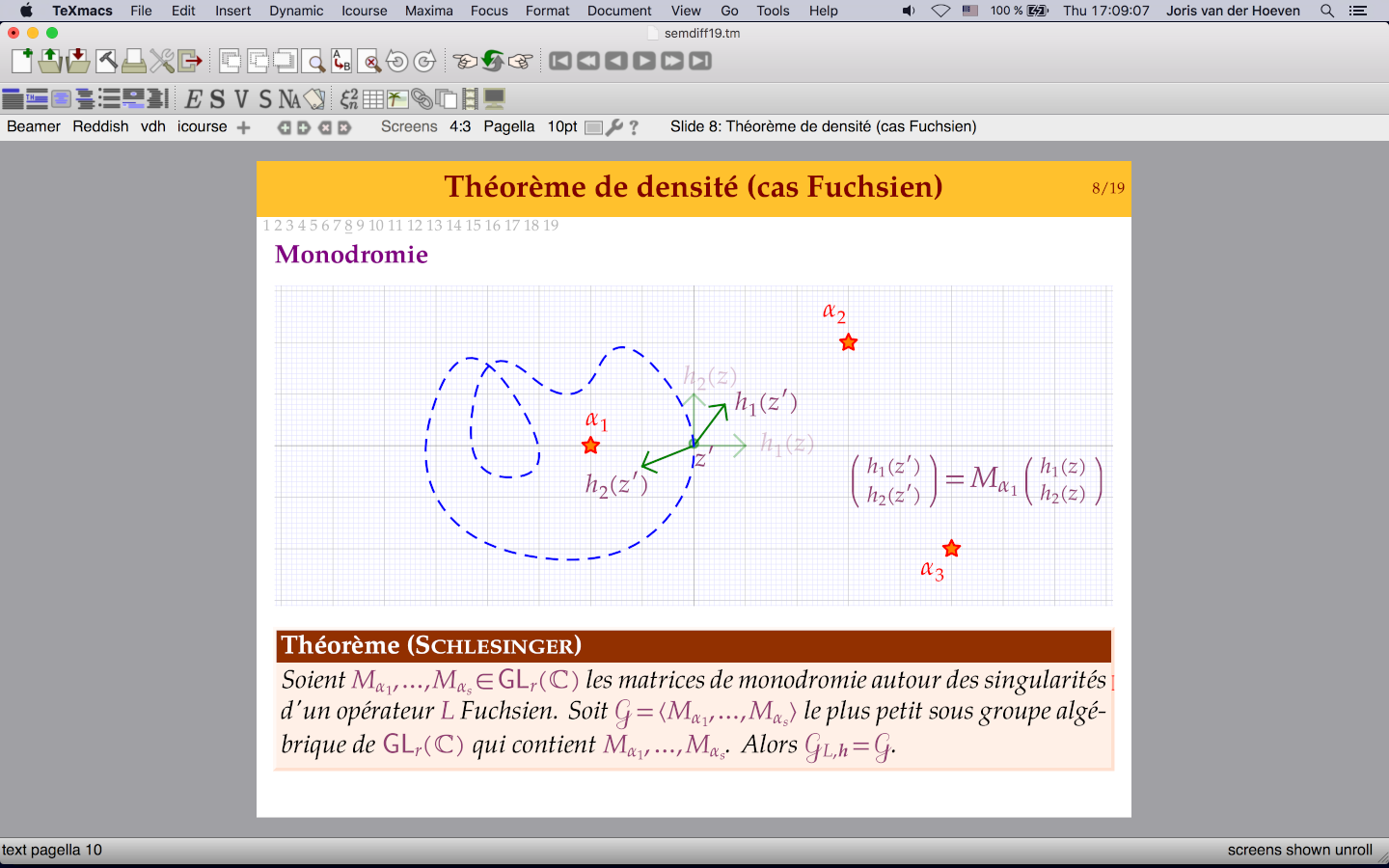
This TCR-like activation induces proliferation, cytokine secretion, and cytotoxicity. The signal travels through the transmembrane domain to the intracellular CD3-zeta costimulatory domain (mainly CD28- or 4-1BB-derived domains). ĬAR T-cells activate when their extracellular domain comprising a single-chain fragment variable (scFv) antibody recognizes the target cell surface antigens. Biological activity is the specific ability or capacity of a product to achieve a defined biological effect. The European Medicine Agency (EMA) defines potency as the measure of biological activity (target-specific cytotoxicity of CAR T-cells) using a bioassay, based on the attribute of the product (target antigen), which is linked to the relevant biological properties (cytotoxicity and related effects). We need to quantify the potency of CAR T-cells using validated assays and good laboratory practices (GLP) before entering into pilot clinical trials (phase 3) to register for the ATMP. Considering high inter-donor or patient variability, a quality control strategy would help the Advanced Therapy Medicinal Product (ATMP) manufacturers to optimize and standardize their manufacturing processes, guaranteeing their reproducibility. These therapies must be subjected to robust quality controls to ensure the safety of each batch and the final product. Five such therapies have been approved by regulatory agencies for blood cancers unresponsive to other treatments, such as B-cell acute lymphoid leukemia, B-lymphoma (diffuse large B-cell and mantle cell lymphoma ), and multiple myeloma. Published by Elsevier B.V.Many chimeric antigen receptor (CAR) T-cell therapies are being developed to treat various cancers. The effective level of isoproterenol and the formation of oxidation products might explain the discrepancies observed in isoproterenol-induced genotoxicity and cytotoxicity.ĭNA strand Breaks Epinephrine Isoprenochrome Isoproterenol Oxidative stress.Ĭopyright © 2022. However, isoprenochrome formation is significantly lower in TexMACS medium. The isoproterenol oxidation product isoprenochrome forms during treatment in both media. Our results show a decrease of isoproterenol concentration in RPMI medium but high stability of the compound in TexMACS medium. However, despite its use in many in vitro and ex vivo studies, the stability of isoproterenol in cell culture media has not been characterized. Clinical studies have shown that isoproterenol is rapidly metabolized in the human body with a plasma half-life of about 2-5 min. Isoproterenol (isoprenaline) is a synthetic catecholamine used as sympathomimetic drug that stimulates β-adrenergic receptors and is widely used in biomedical research. In vitro mechanistic research is mostly performed without taking into consideration the potential influence of cell culture media and/or their supplements and therefore, interactions between compounds of interest and medium ingredients may be overlooked.


 0 kommentar(er)
0 kommentar(er)
